GET LOW I STAY LOW
when switching your patients from another therapy1,2
Real-world IRIS Registry
Data from a real-world analysis of the IRIS Electronic Health Record Registry linked to the Komodo Health Research Dataset (adjudicated pharmacy claims) support switching to VYZULTA for patients receiving PGA and non-PGA IOP-lowering therapies.2
~30% of VYZULTA patients experienced an IOP reduction of ≥5 mmHg2
and
~50% showed an IOP reduction of ≥3 mmHg2
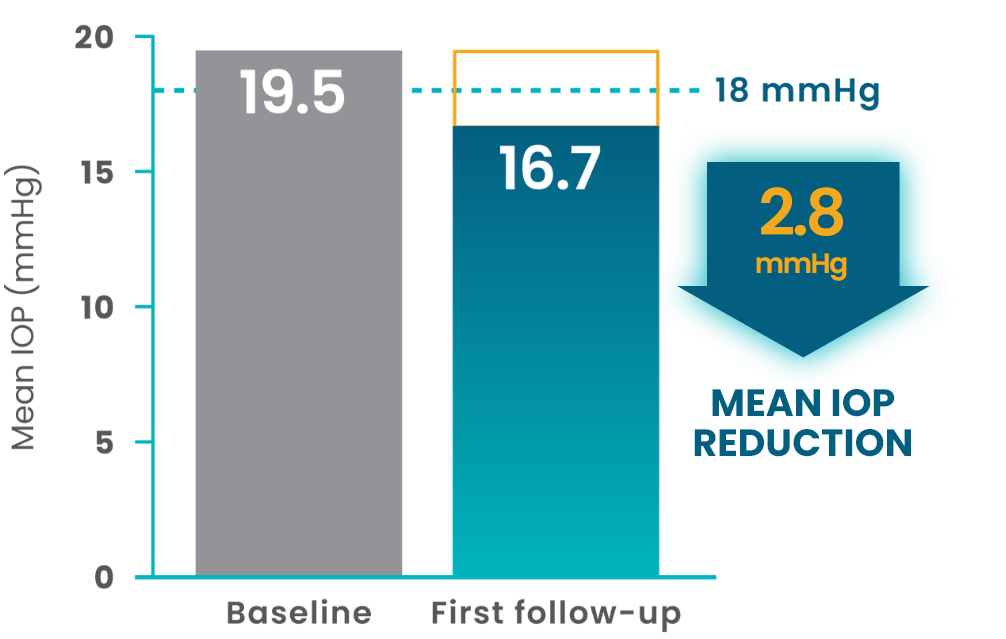
More than 800 patients who switched to VYZULTA showed a mean IOP reduction by first follow-up2
(N=833)
(N=461)
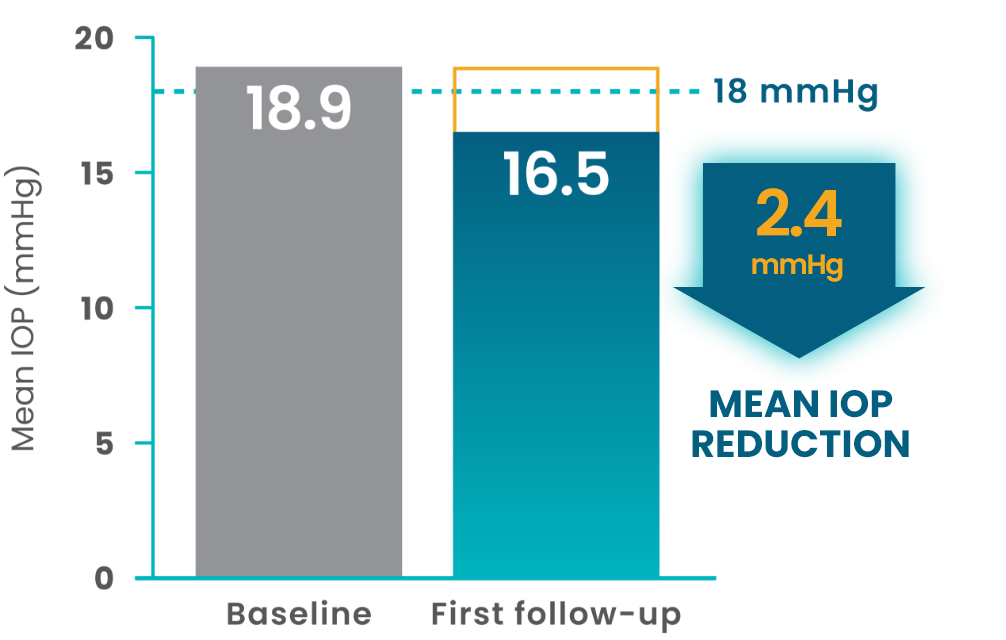
- In the Advanced Glaucoma Intervention Study (AGIS), reduction of IOP below 18 mmHg was associated with maintenance of visual field over long-term follow-up3
- While AGIS did not study VYZULTA specifically, it provides a helpful benchmark to understand the importance of IOP reduction in slowing disease progression
Retrospective 2024 Chart Review
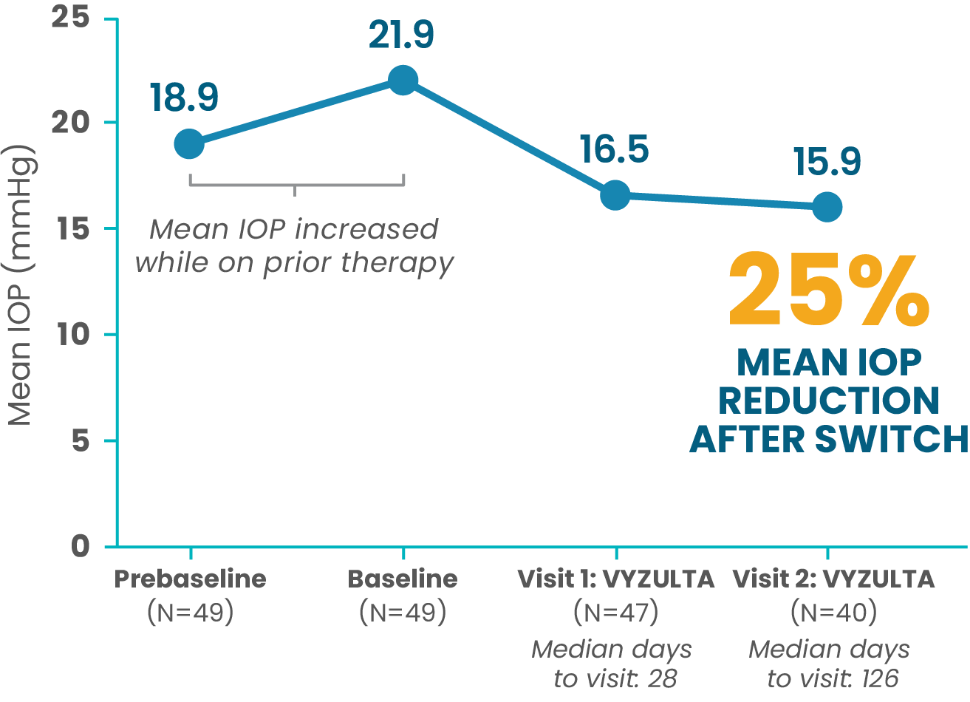
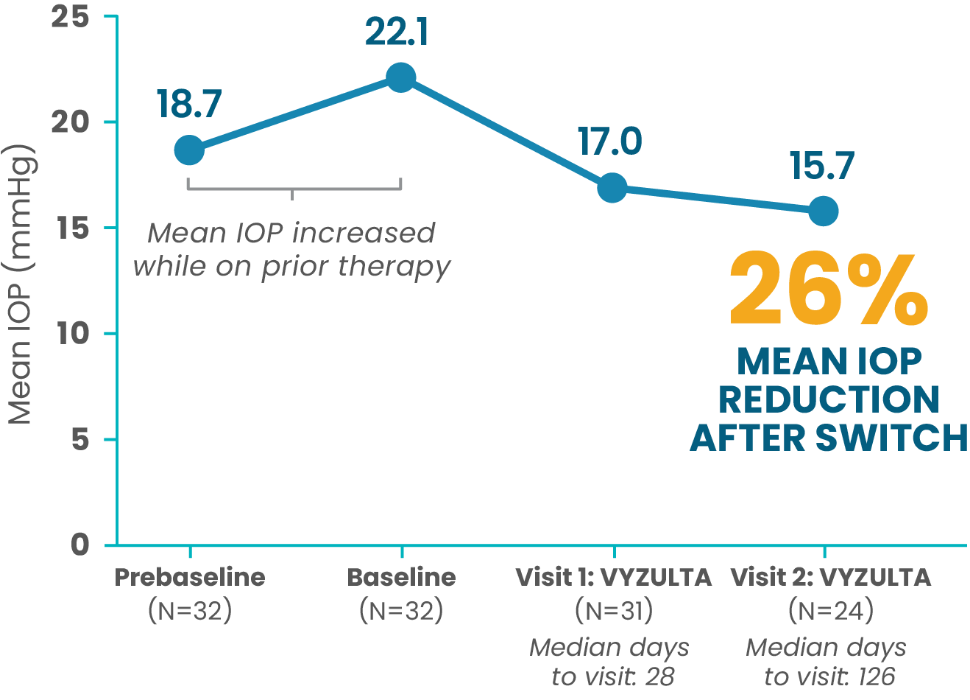
In real-world practice, VYZULTA demonstrated IOP reduction in patients switched from other IOP-lowering therapies.4
All Patients Switched to VYZULTA
Patients Switched to VYZULTA
Following PGA Monotherapy
Prior PGA monotherapy included bimatoprost (n=11),
latanoprost (n=10), travoprost (n=9), and tafluprost (n=2)
3 in 5 patients with mild to moderate glaucoma who switched to VYZULTA showed IOP reduction of ≥5 mmHg from baseline at each follow-up visit4
START LOW I STAY LOW
when choosing a first-line treatment1,5
Retrospective 2020 Chart Review
In real-world practice, VYZULTA demonstrated IOP reduction in patients receiving first-line therapy5
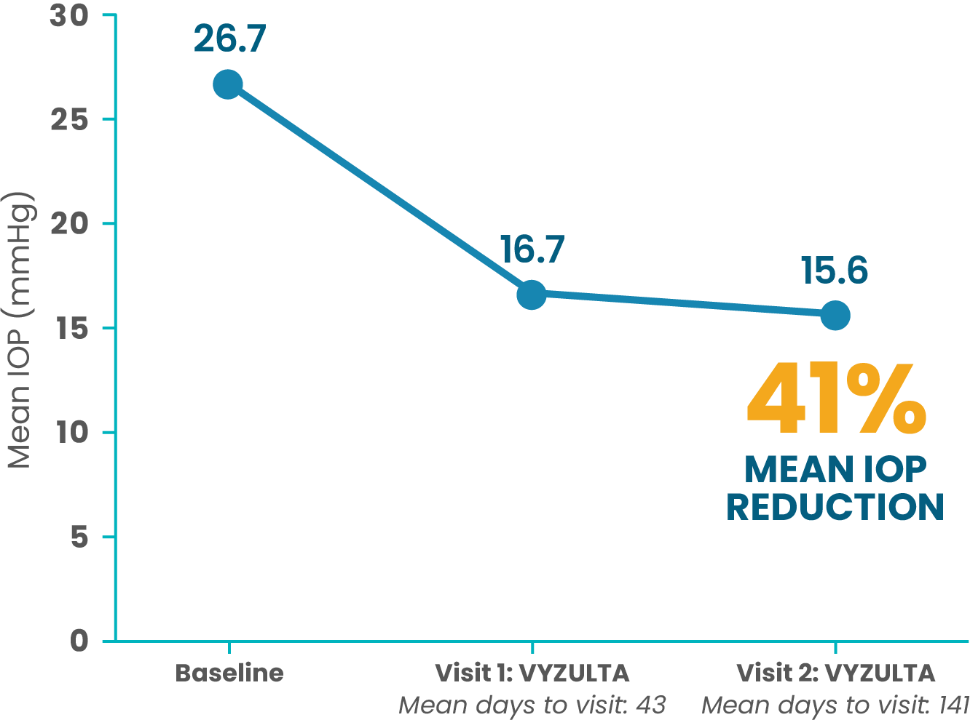
High Baseline IOP (>21 mmHg; N=30)
Low Baseline IOP (≤21 mmHg; N=35)
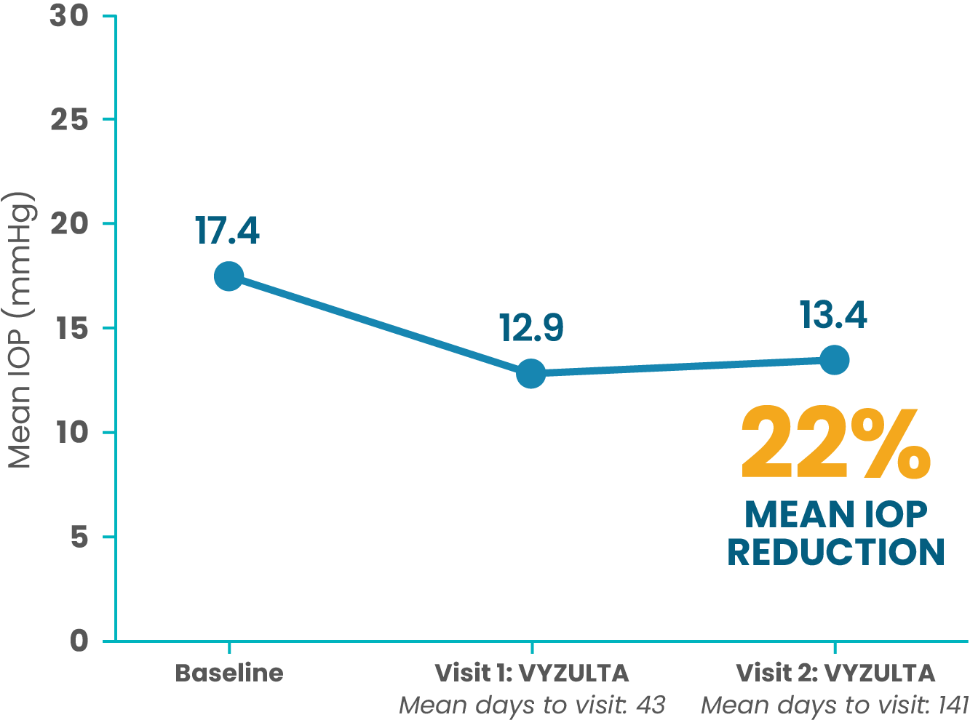
More than half of patients treated first with VYZULTA had ≥30% IOP reduction from baseline at each follow-up visit5
This retrospective chart review reinforces what was seen in clinical trials*
The results of these studies are based on real-world data and should be considered in the context of the limitations of this type of evidence. While these findings provide insights into the real-world performance of VYZULTA, they may not be directly comparable to results from the clinical trials.
*Mean (SD) baseline IOP was 21.7 (5.9) mmHg. A ≥30% IOP reduction was noted in 54.0% and 50.8% of patients at visits 1 and 2, respectively.
IOP=intraocular pressure; PGA=prostaglandin analog.
Sign up
Sign up to receive updates and learn more about VYZULTA.

INDICATION
VYZULTA® (latanoprostene bunod ophthalmic solution), 0.024% is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
IMPORTANT SAFETY INFORMATION
- Increased pigmentation of the iris and periorbital tissue (eyelid) can occur. Iris pigmentation is likely to be permanent
- Gradual changes to eyelashes, including increased length, increased thickness, and number of eyelashes, may occur. These changes are usually reversible upon treatment discontinuation
- Use with caution in patients with a history of intraocular inflammation (iritis/uveitis). VYZULTA should generally not be used in patients with active intraocular inflammation
- Macular edema, including cystoid macular edema, has been reported during treatment with prostaglandin analogs. Use with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema
- There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products that were inadvertently contaminated by patients
- Contact lenses should be removed prior to the administration of VYZULTA and may be reinserted 15 minutes after administration
- Most common ocular adverse reactions with incidence ≥2% are conjunctival hyperemia (6%), eye irritation (4%), eye pain (3%), and instillation site pain (2%)
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here to see full Prescribing Information.
References: 1. VYZULTA. Prescribing Information. Bausch & Lomb Inc. 2. Okeke C, Arkin-Leydig K, Hatfield M, Brevetti TL, Chang RT, Nair AA. Reduction in intraocular pressure after switching to latanoprostene bunod in glaucoma patients in real-world clinical practice. Presented at: Annual Meeting of the American Academy of Ophthalmology; Oct. 18-21, 2024; Chicago, IL. Session PO339. 3. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between the control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2020;10:429-440. 4. Okeke CO, et al. Clin Ophthalmol. 2024;18:409-422. 5. Okeke CO, et al. Ophthalmol Ther. 2020;9(4):1041-1053.
INDICATION
VYZULTA® (latanoprostene bunod ophthalmic solution), 0.024% is indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
IMPORTANT SAFETY INFORMATION
- Increased pigmentation of the iris and periorbital tissue (eyelid) can occur. Iris pigmentation is likely to be permanent

